INTRODUCTION
Existing energy production processes exhibit several unsustainable characteristics such as emissions of greenhouse gases into the atmosphere, and air pollution. Concerns over climate change have fostered increased interest in, as well as development and applications of, clean energy resources such as solar, geothermal, wind, hydro, tidal, biomass.
Research has also been advancing on energy technologies such as those for hydrogen production (Vaghasia et al., 2018). Most presently used techniques for hydrogen production use oil, natural gas, and coal such as steam-methane reforming (SMR). These techniques emit carbon dioxide to the atmosphere, which leads to increased concentrations of greenhouse gases in the atmosphere (Jianu et al., 2013). One of the alternatives to current hydrogen production technology is thermochemical water decomposition, and a particularly promising thermochemical process is the copper-chlorine cycle. A hydrogen economy, using the copper-chlorine cycle for hydrogen production, has the potential to mitigate issues pertaining to climate change, pollution, and the depletion of conventional fuels. Provided the energy input is derived from clean sources, the copper-chlorine cycle is a clean energy alternative as all chemicals involved for hydrogen production are recycled excluding water (Naterer et al, 2011). The applications of hydrogen production range from transportation to manufacturing processes for producing chemicals, food, and electronics. Since hydrogen is widely used a cleaner alternative for its production is in demand (Naterer et al, 2011). A primary challenge for hydrogen as a source of clean energy is its sustainability and an economical method of generating hydrogen in large quantities. Examining the dissolution of cuprous chloride in hydrochloric acid, for various reaction parameters, can assist efforts to optimize the copper-chlorine cycle for hydrogen production (Vaghasia et al., 2018; Jianu et al., 2013).
In this study, an experimental design is developed to aid in the development of an empirical model of dissolution rates when concentration is varied. In this article, the experimental design is described. The reaction parameters observed in the experimental design include quantity of cuprous chloride, and concentration of hydrochloric acid, as well as electrical conductivity, ambient temperature, pH, and photosensitivity of cuprous chloride in aqueous hydrochloric acid solution. As hydrochloric acid is added to the previously washed in distilled water cuprous chloride, the colour of the solution changes gradually. This qualitative change is detected by a light dependent resistor (LDR) as a beam of light passes through the solution onto a light dependent resistor.
THEORETICAL BACKGROUND AND LITERATURE REVIEW
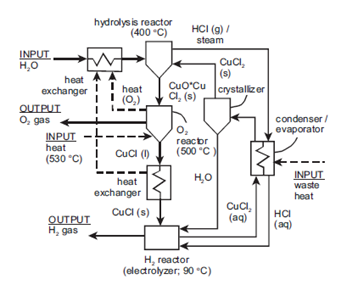
A promising technology for large scale production of hydrogen are thermochemical water-splitting cycles. In thermochemical cycles the intermediate compounds are recycled within a closed loop. This decreases the amounts of chemicals and pollutants released (Jianu et al., 2013). Iron oxide cycle, copper-chlorine cycle, hybrid sulfur cycle, sulfur-iodine cycle, zinc-zinc oxide cycle, and cerium (IV) oxide-cerium (III) oxide cycle are few of the thermochemical cycles proposed for hydrogen production. However, most of these cycles are not feasible on a substantial scale as the temperature requirements are relatively higher compared to the copper-chlorine cycle. The copper-chlorine cycle requires temperatures up to 530 °C (Jianu et al., 2018). The copper-chlorine cycle also has the ability to use low-grade waste heat to improve energy efficiency and has low voltage requirements for the electrochemical step (Jianu et al., 2016).
The copper-chlorine cycle is a four-step thermochemical water decomposition process. The steps include electrolysis, drying, hydrolysis, and thermolysis. Water is decomposed into hydrogen and oxygen using intermediate copper and chlorine compounds. The reactions form a closed loop that has the net effect of splitting water into hydrogen and oxygen, while recycling all other chemicals (Diffenderfer, 2005). The ternary steps of this system involve mass transfer in the form of dissolution (Jianu et al., 2013; Jianu et al., 2018; Jianu et al., 2016). The dissolution of cuprous chloride in hydrochloric acid is a ternary step that occurs between the thermolysis and electrolysis steps in the overall cycle.
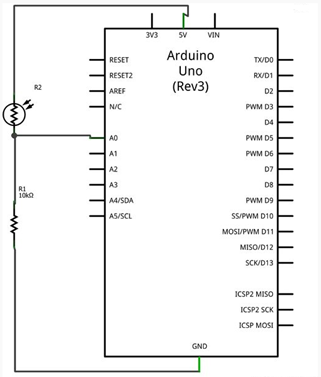
The refractive index is the primary principle for the analysis of dissolution of cuprous chloride in aqueous hydrochloric acid in this experimental design. In optics, the index of refraction describes how light propagates through a medium. The laser shines through the solution onto a light dependent resistor (LDR). A light dependent resistor or a photoresistor is a light-controlled variable resistor (Diffenderfer, 2005). The resistance of the light dependent resistor decreases with increasing light intensity. In order to be able to read the data from the light dependent resistor an Arduino has be to be used. The Arduino Uno acts as a median between the data sent from the sensor to the computer. An Arduino can only measure in terms of voltage which is achieved by connecting the light dependent resistor and a standard resistor in a potential divider circuit. Ohm’s law is described by the equation I = V/R, where I is the current in amperes, V is the voltage in volts, and R is the resistance in ohms (Hambley, 2010). According to Ohm’s law, current is directly proportional to voltage, which implies that voltage is directly proportional to resistance if current is kept constant. Therefore, as the light intensity decreases the output voltage from the light dependent resistor increases. The amount of light absorbed by the solution of cuprous chloride in aqueous hydrochloric acid is quantified by the voltage of the Arduino connected to the light dependent resistor.
EXPERIMENTAL DESIGN AND PROCEDURE
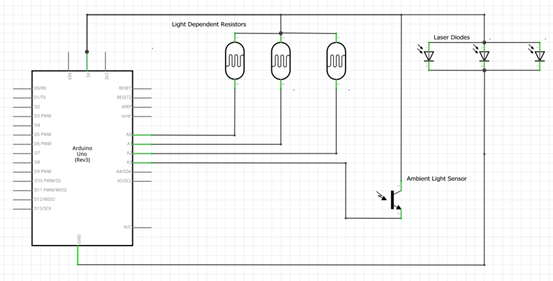
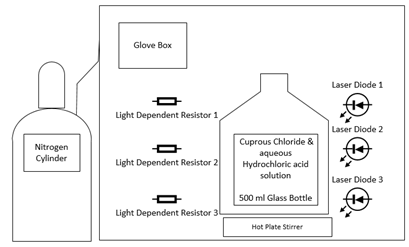
The proposed experimental design requires an Arduino Uno, three red lasers, three twelve millimeter light dependent resistors, nitrogen cylinder, red light filter, ambient light sensor, and conductivity meter. Three light dependent resistors are chosen for the purpose of this experimental design to prevent premature detection of local saturation. The ancillary apparatus required for the experiment includes the following: laser mounts, 3D printed stands for the laser and the light dependent resistors, a breadboard for mounting sensors, jumper cables and wires, a 500 milliliter glass bottle with a closed top, a glove box, a hot plate stirrer, which is incorporated into the experimental design to speed up dissolution of cuprous chloride in aqueous hydrochloric acid, and a nitrogen gas cylinder and regulator. In the experimental design, the controlled variables are the ambient temperature when the concentration of the hydrochloric acid is varied, constant supply of nitrogen in the glove box, and the quantity of cuprous chloride in aqueous hydrochloric acid, i.e. 500 milliliters. The independent variables include the ambient temperature, concentration of hydrochloric acid, and amount of cuprous chloride. The dependent variables are the reaction time and the amount of light absorbed in millivolts.
The experiment is to be conducted in the glove box. Before commencing the experiment, the glove box is purged with nitrogen since cuprous chloride is air and light sensitive. The electrical conductivity is measured before and after dissolution of cuprous chloride in hydrochloric acid. Total dissolved salts (TDS) can be obtained from the electrical conductivity of the solution. Three lasers and light dependent resistors are mounted on identical stands on opposite sides of the 500-millilitre glass bottle. Multiple light dependent resistors are used to monitor the reaction gradient. The ambient light sensor is used to normalise the data collected from the light dependent resistor, as they are extremely sensitive to changes in light. The experiment commences at the ambient temperature of 20 °C. The first run begins with 10 grams of cuprous chloride and 1M hydrochloric acid. The ambient temperature and the concentration of hydrochloric acid are kept constant while the quantity of cuprous chloride is varied from 10 grams to 30 grams in increments of 10 grams. This amounts to three runs with varying quantities of cuprous chloride. The ambient temperature and the quantity of cuprous chloride are kept constant while the concentration of hydrochloric is varied from 1M to 9M in increments of 3M. This amounts to a cumulative total of twelve runs with varying concentrations of both hydrochloric acid and cuprous chloride. The concentration of hydrochloric acid and the quantity of cuprous chloride are then kept constant while the ambient temperature is varied from 20 °C to 35 °C in increments of 5 °C. This amounts to a total of forty-eight runs. Each run is deemed complete once the voltage levels of the three light dependent resistors stabilize to an equal value within the acceptable tolerance. The tolerance of the system is limited by the resolution of the microprocessor, ATmega328/P, and the supply voltage to the light dependent resistor from the Arduino, 5 volts. Therefore, the tolerance is ± 5/210 which is ± 0.012 volts.
As the solution of cuprous chloride in aqueous hydrochloric acid gets darker in colour, it is assumed that saturation is approaching and the reaction time will be recorded. Light intensity is inversely proportional to the resistance recorded by the light dependent resistor. Thus, resulting in a lower output voltage from the light dependent resistor. X-ray diffraction (XRD) analysis for the cuprous chloride sample is performed and will be compared with theoretical data as the sample over time could have been exposed to ambient air. Once the reaction time, and the amount of light intensity absorbed in millivolts is collected, and the total dissolved salts (TDS) from electrical conductivity in siemens per meter is calculated, alongside the varied quantity of cuprous chloride and ambient temperature, the analysis of variance (ANOVA) can be performed. Analysis of variance is a collection of statistical models commonly used to analyse experimental data. Analysis of variances suggests correlations among various experimental parameters. Analysis of variance can then be used to extrapolate and or forecast the approximate reaction time on a larger scale. This empirical model can assist in reducing chemical waste as analysis of variance can be used to extrapolate and or forecast the approximate reaction time, thus avoiding saturation. This model would also reduce residual deposition since, in a closed loop cycle such as the thermochemical copper-chlorine cycle, residual of undissolved compounds can introduce impurities into the system.
CONCLUSION
A novel procedure for monitoring the dissolution of CuCl in HCl is presented. The procedure is based on light intensity of the solution as the concentration of CuCl is increased. The light intensity would be correlated to the solution temperature and its concentration to determine the dissolution rate and solubility equilibrium. The solubility equilibrium is expected to aid in the integration of the thermolysis and the electrolysis stage as the copper-chlorine thermochemical water decomposition cycle.
ACKNOWLEDGEMENTS
Support of this research from Canadian Nuclear Laboratories, the Ontario Research Excellence Fund, and the Natural Sciences and Engineering Research Council of Canada is gratefully acknowledged.

 Full Text (PDF)
Full Text (PDF)